Policy Actions
Innovative animal drugs products are needed to address serious animal diseases, including epilepsy, asthma, arthritis, cardiac disease, tick borne illnesses like Lyme Disease, feline infectious anemia, Johne’s disease and blackhead disease.
To provide the necessary medicines to keep animals healthy, the regulatory processes to approve medicines must be efficient and Congress must appropriately fund the offices within the FDA and EPA that oversee the development, approval, and administration of animal medicines.
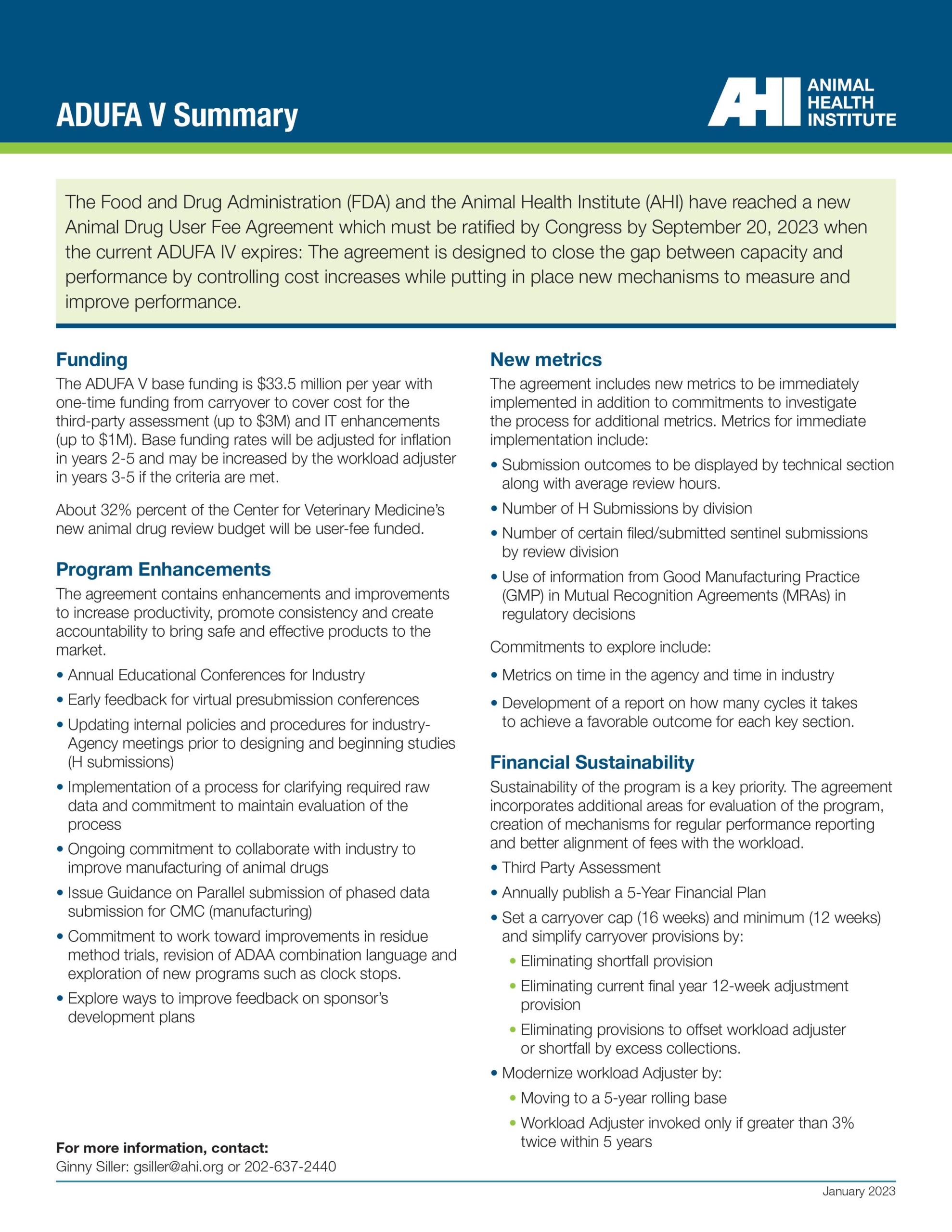
The Animal Drug User Fee Act (ADUFA) and the Pesticide Registration Improvement Act (PRIA) establish fees paid by the companies that develop animal medicines while also funding the agencies that oversee animal medicines.
PRIA was reauthorized in December 2022, but ADUFA is set to expire in October 2023.
ADUFA is authorized five years at a time, and its approval traditionally receives bi-partisan Congressional support.
AHI Testifies Before the Energy & Commerce Committee